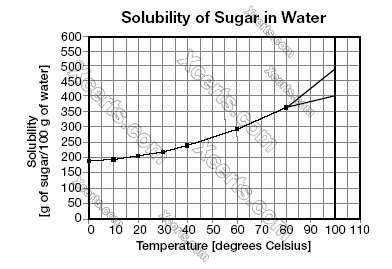
A mixture that is made by dissolving one compound (solute) in another (solvent) is called a solution. The amount of solute that can be dissolved in a solvent at a given temperature is called solubility. For most substances, solubility increases with temperature. When the amount of solute dissolved in a solvent exceeds the solubility, the solution is called supersaturated. Rock candy can be made by dissolving as much sugar in water, as solubility would allow at a high temperature, and then slowly cooling the solution to room temperature. If a thin string is dipped into it and left in the solution, the sugar in excess of the solubility at room temperature will form sugar crystals around the string, making the sweet rock candy. The solubility (in grams of sugar per 100 grams of water) as a function of temperature (in degrees Celsius) is plotted in the graph below.
In a solution of sugar and water, which is the solvent and which is the solute?
- solvent: sugar; solute: water
- solvent: rock candy; solute: water
- solvent: water; solute: sugar
- solvent: water; solute: rock candy
Answer(s): C
Explanation:
According to the passage, the compound that is dissolved is the solute, while the liquid is the solvent.
Therefore, in sugar water, sugar is the solute and water is the solvent.